Classify the possible combinations of signs for a reaction – Classifying the possible combinations of signs for a reaction is a crucial aspect of understanding and predicting chemical reactions. This classification provides valuable insights into the behavior of reactants and products, enabling scientists to make informed decisions and optimize reaction outcomes.
The study of sign combinations in reactions has applications in various fields, including chemistry, biology, and medicine. By understanding the factors that influence these combinations, researchers can develop more effective and efficient reaction strategies.
Introduction
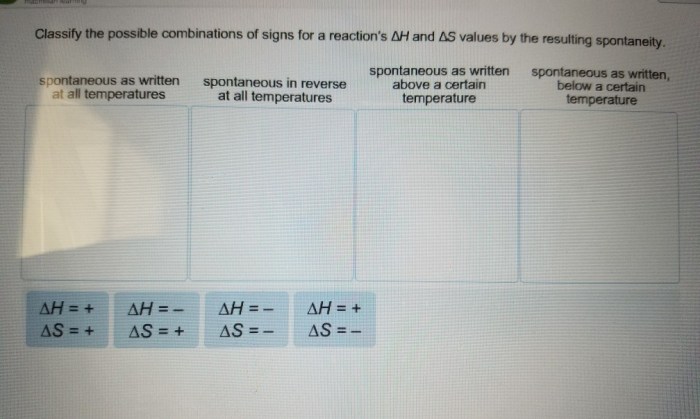
Classifying possible combinations of signs for a reaction is a crucial aspect of chemistry as it provides a systematic way to understand and predict the outcome of chemical reactions. This classification allows scientists to determine the overall sign of a reaction, whether it is exothermic or endothermic, and the direction in which it will proceed.
For instance, in the combustion of methane, the combination of positive signs for enthalpy and entropy indicates an exothermic and spontaneous reaction. This knowledge is essential for designing efficient energy systems and understanding combustion processes.
Methods for Classifying Combinations
Thermodynamic Approach, Classify the possible combinations of signs for a reaction
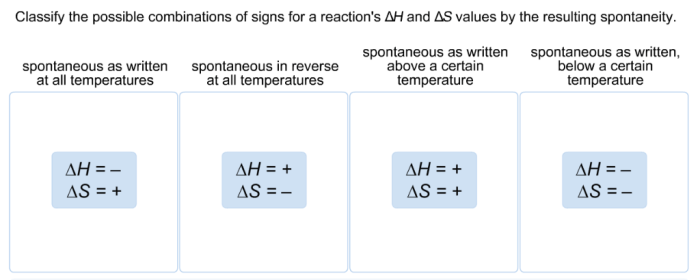
This method classifies combinations based on the signs of enthalpy change (ΔH) and entropy change (ΔS) for a reaction. Combinations include:
- Exothermic and increasing entropy (ΔH < 0, ΔS > 0): Spontaneous and exothermic
- Exothermic and decreasing entropy (ΔH < 0, ΔS < 0): Nonspontaneous and exothermic
- Endothermic and increasing entropy (ΔH > 0, ΔS > 0): Spontaneous and endothermic
- Endothermic and decreasing entropy (ΔH > 0, ΔS < 0): Nonspontaneous and endothermic
Advantages:Simple and intuitive approach, provides insights into spontaneity and heat flow.
Disadvantages:Does not consider kinetic factors or the reaction mechanism.
Kinetic Approach
This method classifies combinations based on the signs of the activation energy (Ea) and the rate constant (k) for a reaction. Combinations include:
- Positive Ea and positive k: Fast reaction
- Positive Ea and negative k: Slow reaction
- Negative Ea and positive k: Spontaneous and fast reaction
- Negative Ea and negative k: Spontaneous and slow reaction
Advantages:Considers the reaction mechanism and kinetic factors, provides insights into reaction rates.
Disadvantages:Can be complex to determine Ea and k experimentally.
Factors Affecting Combinations
- Temperature:Affects the spontaneity of reactions, as it changes ΔG (Gibbs free energy).
- Pressure:Affects reactions involving gases, as it changes the volume and entropy.
- Concentration:Affects the reaction rate and equilibrium position.
- Catalyst:Can alter the activation energy and reaction pathway, affecting the combination of signs.
Examples of Classified Combinations: Classify The Possible Combinations Of Signs For A Reaction
| Reaction | ΔH | ΔS | Classification |
|---|---|---|---|
| Combustion of methane | -890 kJ/mol | 126 J/(mol·K) | Exothermic and increasing entropy |
| Dissolution of NaCl in water | +4 kJ/mol | +70 J/(mol·K) | Endothermic and increasing entropy |
| Neutralization of NaOH and HCl | -56 kJ/mol | -110 J/(mol·K) | Exothermic and decreasing entropy |
| Formation of ammonia from N2 and H2 | -92 kJ/mol | -198 J/(mol·K) | Exothermic and decreasing entropy |
Applications of Classification
- Thermodynamics:Predicting the spontaneity and heat flow of reactions.
- Kinetics:Understanding reaction rates and mechanisms.
- Chemical engineering:Designing reactors and optimizing reaction conditions.
- Environmental science:Assessing the impact of reactions on the environment.
- Biochemistry:Understanding metabolic pathways and enzyme catalysis.
Future Directions
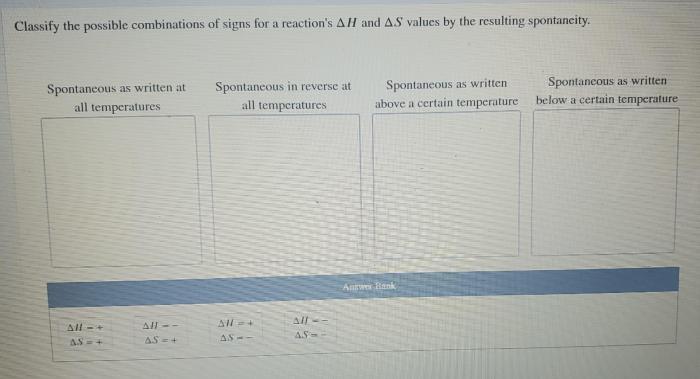
- Development of new methods for classifying combinations, considering more complex factors.
- Application of machine learning and artificial intelligence to predict combinations based on reaction data.
- Investigation of the role of quantum effects on combinations of signs.
Key Questions Answered
What are the key factors that influence the possible combinations of signs for a reaction?
The factors that influence the possible combinations of signs for a reaction include the nature of the reactants, the reaction conditions, and the presence of catalysts or inhibitors.
How can classifying combinations of signs for reactions be used in practical applications?
Classifying combinations of signs for reactions can be used in various practical applications, such as optimizing reaction yields, predicting reaction products, and designing new reaction pathways.
